Preimplantation genetic testing for aneuploidies (PGT-A, previously known as preimplantation genetic screening or PGS) provides information on the chromosomal content of cell samples from blastocysts with the intent of helping inform decisions about embryo transfer.
PGT-A was initially designed to classify IVF embryos as euploid (containing the correct amount of chromosomal material) or aneuploid (containing extra or missing chromosomal material) because of the known association between advanced maternal age and increased occurrence of aneuploidy. However, recent advancements in PGT-A technology have expanded the range of possible results to include an intermediate between euploid and aneuploid: mosaic, raising new questions about PGT-A and the clinical impact of mosaicism.
Evidence for PGT-A
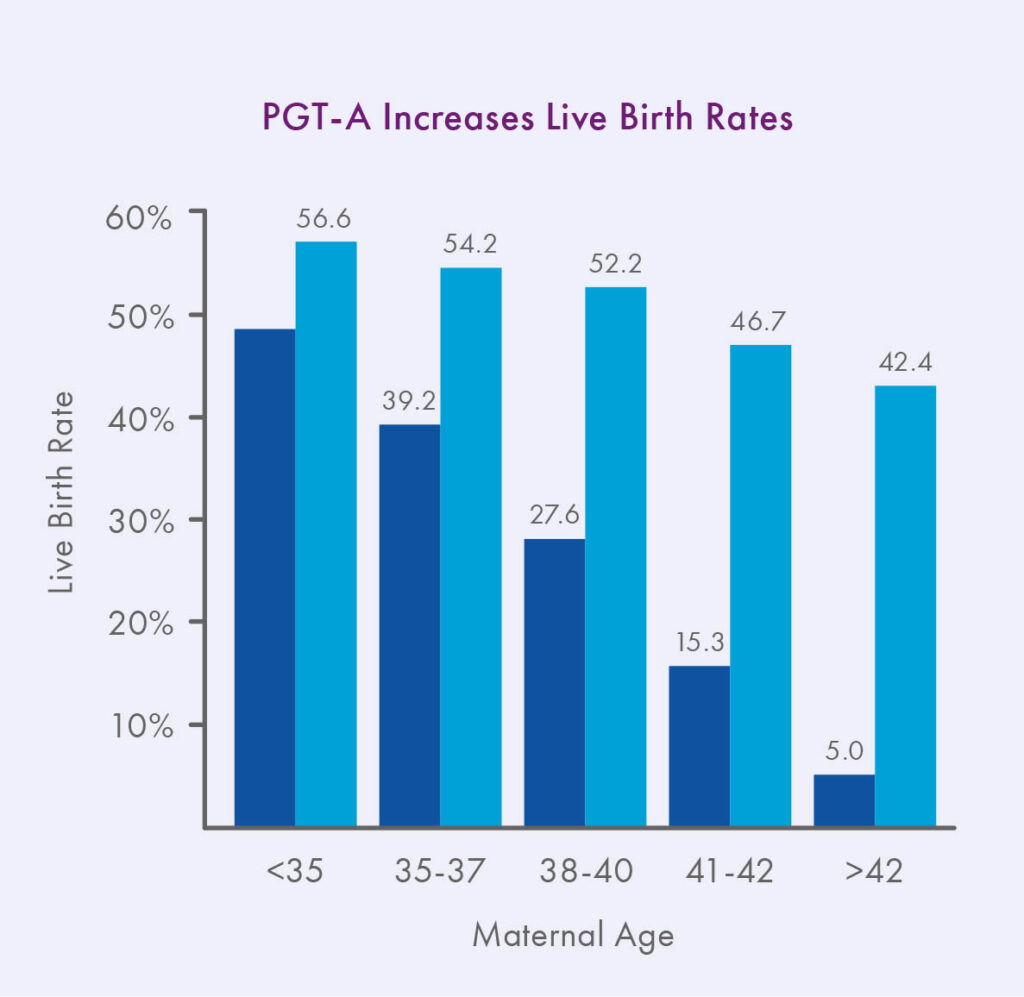
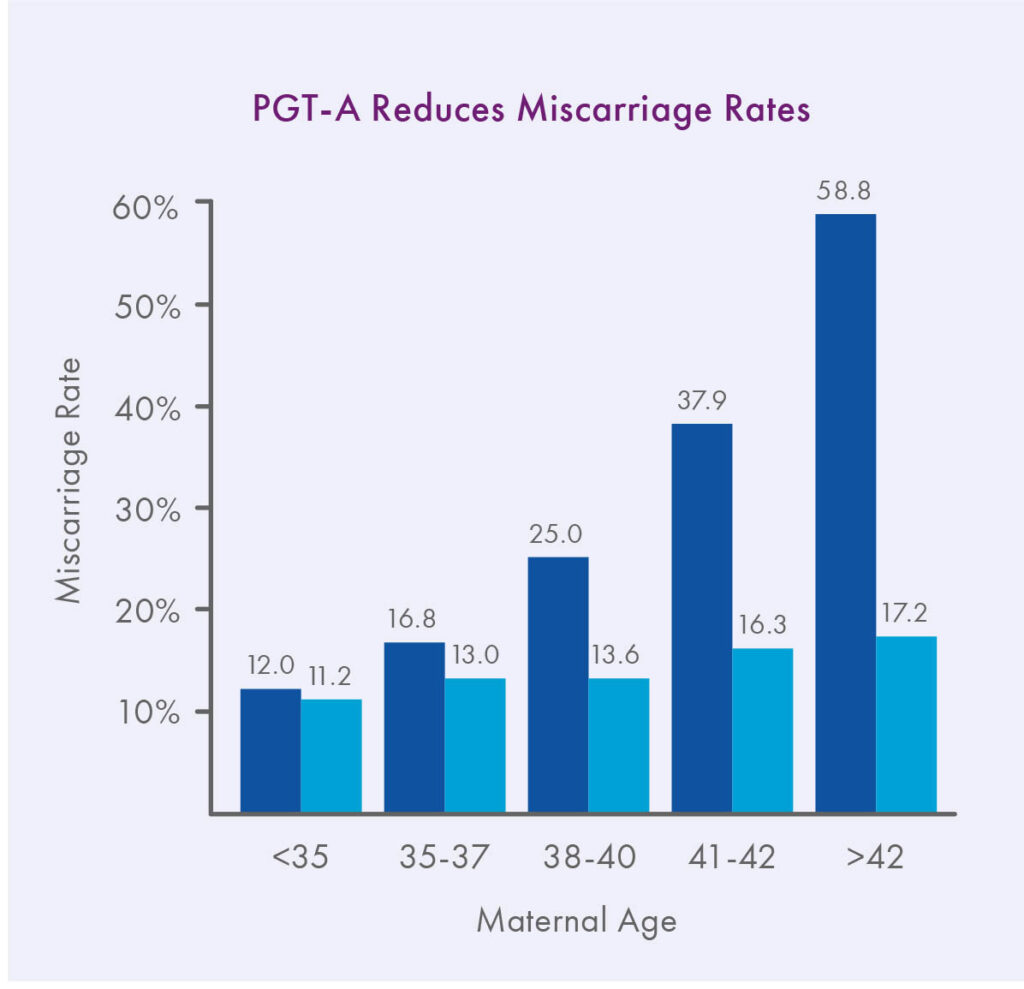
As first reported in Harton et al. 2013 and now also observed in the latest SART data, IVF with PGT-A can effectively reverse the maternal age effect on implantation rates, especially for frozen transfers.¹ In addition, since the advent of PGT-A, the available scientific evidence is overwhelming that euploid embryos have lower miscarriage rates and higher ongoing pregnancy rates per transfer than embryos replaced at random, at least for patients 35 and older.2-6
Because of the increased likelihood of success a tested euploid embryo has over an untested embryo, PGT-A has also enabled a shift from multiple embryo transfer to single embryo transfer, reducing patients’ risk of complications associated with twin and other multiple gestations.³
While the data on euploid embryo transfer is robust and conclusive, the research on mosaic embryos continues to evolve.
Mosaicism: What It Is and How It Happens
In the context of PGT-A, mosaicism is understood as an embryo containing two or more distinct cell lines; the example most relevant here is an embryo containing a mix of euploid and aneuploid cells. Although the concept of mosaicism has been recognized in biology for some time, it is only with the increased sensitivity of some next-generation sequencing (NGS) technology platforms that it has been reliably detected in preimplantation embryos. While aneuploidy in an entire embryo typically arises as a result of meiotic errors during gamete formation, mosaicism arises through mitotic errors during embryo division. As mosaicism is a result of mitotic, not meiotic, errors, it has not been shown to be associated with maternal age.7
The Clinical Relevance of Mosaicism
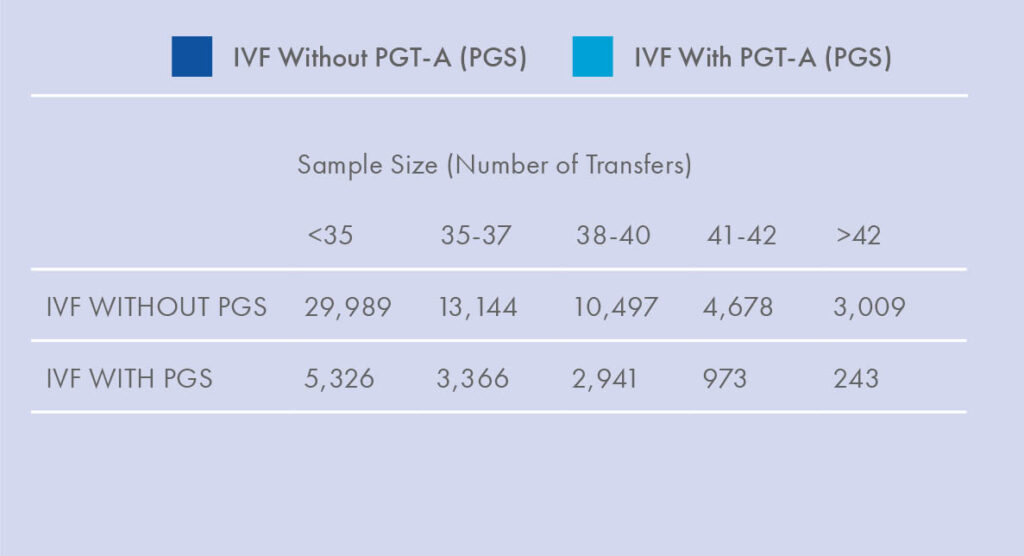
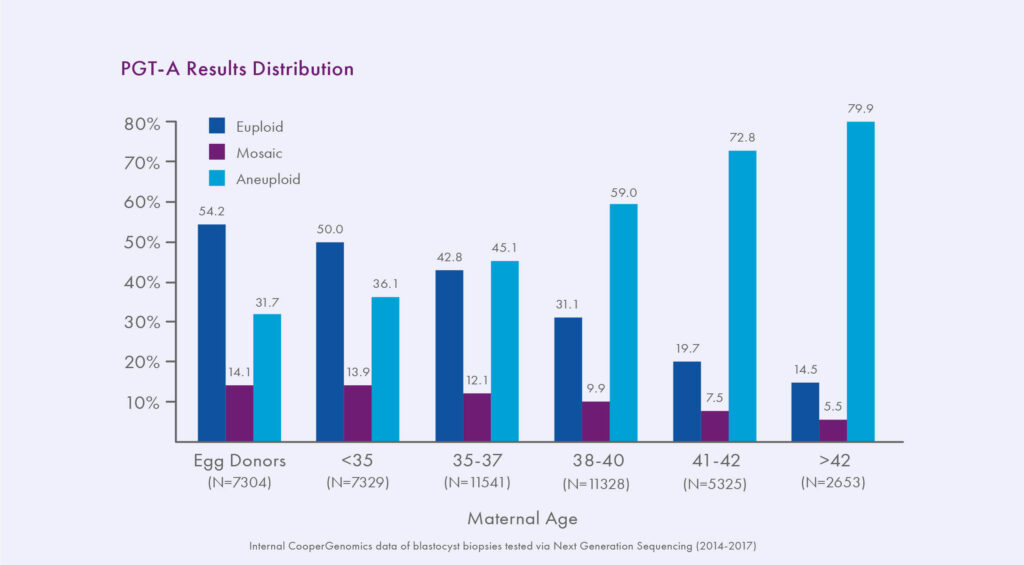
About 10-20% of PGT-A results using high resolution NGS techniques are mosaic.3, 7-10 Mosaicism has always existed in embryos, but has only recently become detectable due to technological advancements. PGT-A Results Distribution Previous methodologies only classified embryos as euploid or aneuploid, we have been unknowingly transferring mosaic embryos for years. Mosaic embryos have been shown to implant less and miscarry more than euploid embryos, but they can still sometimes lead to healthy live birth.3, 7-10 Indeed, as an in-between result, mosaic embryos’ chance of producing a viable pregnancy is intermediate between that of a euploid embryo and an aneuploid embryo. A recent internal study compared three key indicators of embryo viability − implantation, miscarriage, and ongoing pregnancy rates − between different groups of embryos; the results indicated viable potential for mosaic embryos, though significantly different from that of euploid embryos. Embryos with a low level of mosaicism (<40% abnormality detected) had an ongoing implantation rate (OIR) of 50%, compared to 30% for higher level mosaics, which was significant. Overall, mosaic embryos had a 24% miscarriage rate, compared to 7% for euploid embryos (p><0.001), and an OIR of 37% per cycle of mosaic (20-80% abnormal) embryo transfer, compared to 77% after euploid embryo transfer (p><0.001).
Society Guidelines
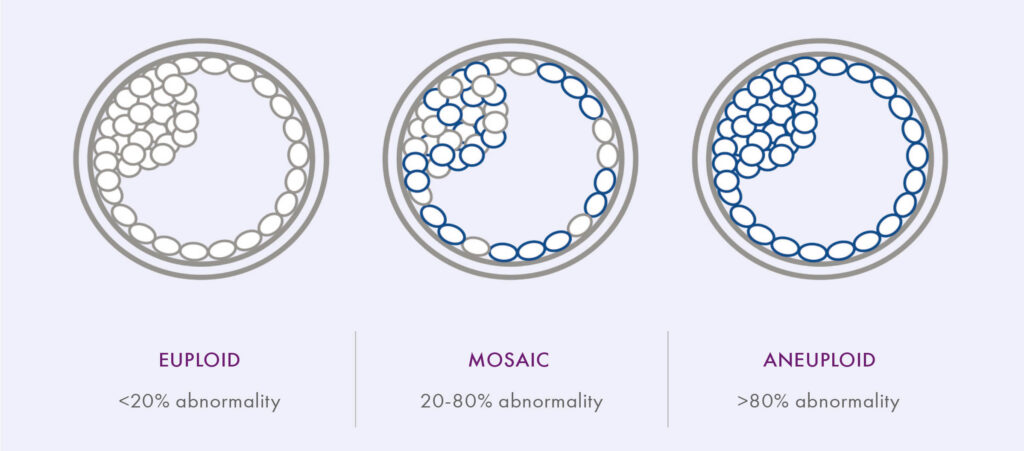
To date, only two societies have issued guideline statements regarding mosaic embryos and recommendations for clinical practice. The first guidelines were shared by the Preimplantation Genetic Diagnosis International Society (PGDIS) in July of 2016; when reporting PGT-A results, the guidelines suggested a ‘cut-off point’ at >20% abnormality detected. Lower levels (<20%) would thus be classified as normal (euploid), >80% abnormal (aneuploid), and the range between 20-80% mosaic.11
At this stage, outcomes data from the transfer of known mosaic embryos was very limited and further recommendations were made on the knowledge obtained from reproductive outcomes where fetal or placental mosaicism had been identified. These chromosome-based recommendations suggested de-prioritization of mosaicism involving chromosomes 2, 7, 13, 14, 15, 16, 18, and 21 based upon their impact on live birth and pregnancy outcomes.
When the Congress on Controversies in Preconception, Preimplantation, and Prenatal Genetic Diagnosis (COGEN) issued their statement soon after, knowledge had already evolved to add an additional comment on the impact the level of mosaicism (low degree versus high degree) can have on an embryo.12 More recently, a study published in Reproductive BioMedicine Online considered four parameters to determine a ‘scoring system’ for embryos based on chromosome change, including the risk for fetal involvement, uniparental disomy, miscarriage, and viable aneuploidy. The result was a comprehensive breakdown of which chromosomes and in what configuration (trisomic versus monosomic) to prioritize for transfer.13 While complicated at first glance, such ‘personalized’ recommendations are where the ultimate answer may lay as we learn more and more about outcomes of known mosaic embryo transfers.
PGDIS Guidelines
References
1.Harton et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril (2013).
2. Forman, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril (2013).
3. Munné et al. Detailed investigation into the cytogenic consititution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil Steril (2017).
4. Rubio et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril (2017).
5. Scott et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril (2013).
6. Yang et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet (2012).
7. Munne & Wells. Detection of mosaicism at blastocyst stage with the use of high-resolution next-generation sequencing. Fertil Steril (2017).
8. Fragouli et al. The developmental potential of mosaic embryos. Fertil Steril (2015).
9. Fragouli et al. Analysis of implantation and ongoing pregnancy rates following the transfer of mosaic diploid-aneuploid blastocysts. Hum Genet (2017).
10. Greco et al. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med (2015).
11. PGDIS Position Statement on Chromosome Mosaicism and Preimplantation Aneuploidy Testing at the Blastocyst Stage. http://www.pgdis.org/docs/newsletter_071816.html (2016).
12. COGEN Position Statement on Chromosomal Mosaicism Detected in Preimplantation Blastocyst Biopsies. http://www.ivf-worldwide.com/
cogen/general/cogen-statement.html (2016).
13. Grati et al. An evidence-based scoring system for prioritizing mosaic aneuploid embryos following preimplantation genetic screening. RBMO (2018).