SAGE 1-Step™ GM-CSF Culture and Transfer Medium

Providing a new opportunity for patients in need
Based on the long-established and clinically proven SAGE 1-Step medium, SAGE 1-Step GM-CSF medium sets a new standard as the first single-step culture and transfer medium containing hyaluronan and the GM-CSF cytokine.
Availability
This product is available for sale in selected countries worldwide.
CONTACT USClinical research underpins diverse
benefits of GM-CSF
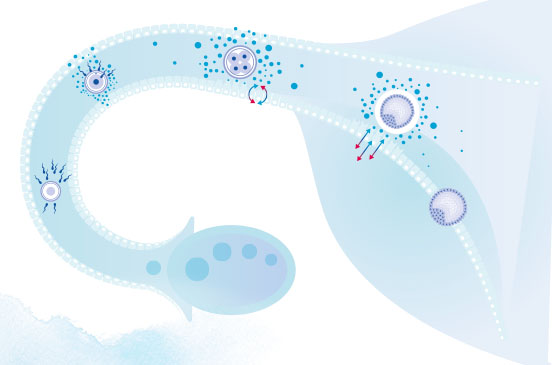
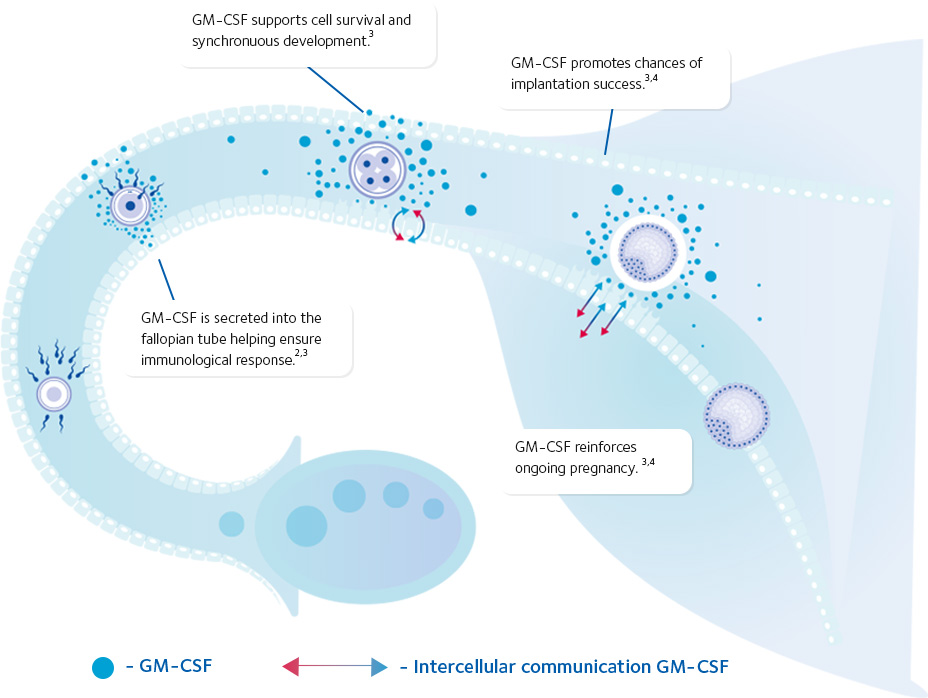
- Mediates embryo-endometrial communication1
- Facilitates successful embryo development2
- Protects the embryo from cell stress3
- Promotes chances of implantation success4
- Enhances the immunological response for implantation5
- Reinforces ongoing pregnancy6
An advanced treatment option for
culture and transfer


Post-warming culture and transfer

Fresh culture and transfer cycles
For patients in need of personalized treatment solutions

Previous Miscarriage

Implantation failure

Advanced maternal age
GM-CSF: the great communicator
Collectively, the research shows GM-CSF’s signaling in vivo plays a crucial role in facilitating successful implantation, embryo development,
promoting enhanced immune response1 and protecting the embryo from cell stress.3

The Past, Present and Future of GM-CSF
with the pioneer of GM-CSF-supplemented culture media.
Watch the masterclass hereDesigned for success
GM-CSF and its role in reproduction have been researched since the 1970s. The past 30 years of research have led to its inclusion in ART media as multiple studies have revealed its impact on clinical outcomes, particularly for some subsets of women.

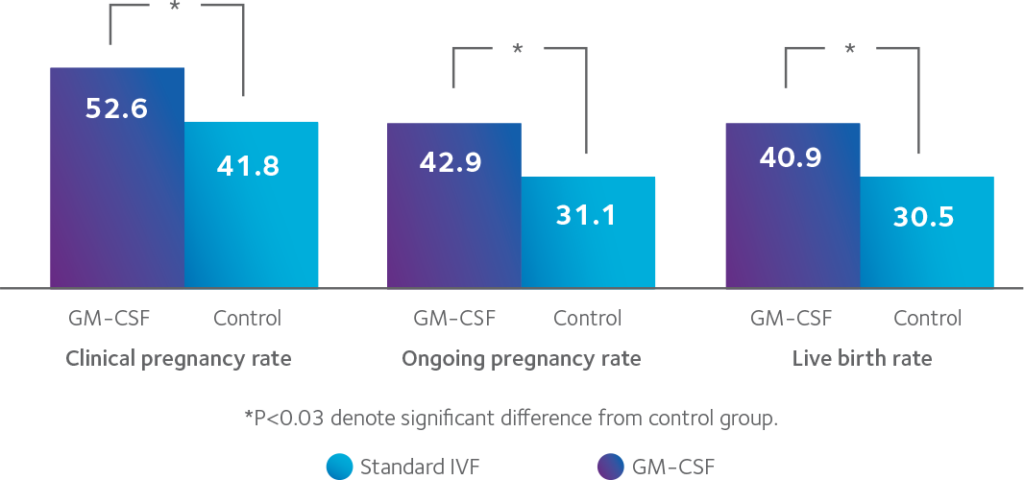
Increased implantation rate for frozen embryo transfer with SAGE 1-StepTM Medium
The use of SAGE 1-StepTM GM-CSF Medium in post-warming culture and transfer, improved the live birth rate as a result of increased implantation rate in the
frozen-thawed blastocyst-transfer cycle.5
Overall and embryo-transfer outcomes (%)
GM-CSF-containing medium vs. Control medium

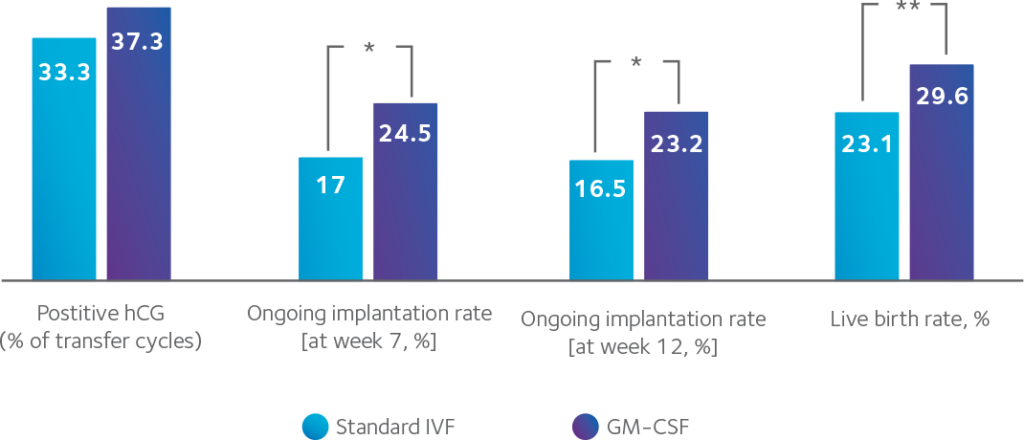
Medium containing GM-CS is an effective treatment option that may improve outcomes for miscarriage patients
In women with previous miscarriage, GM-CSF can increase ongoing implantation rates.
Impact of culture medium containing GM-CSF on previous miscarriage patients6
Helpful resources

Less paper, more languages
To reduce our environmental impact and to support efficiency, we have introduced electronic IFUs (eIFU). Our eIFUs allow you to access important product information in multiple languages without having to store lots of printed materials. Get the information you need quickly, and in the language you need

Product Specification
GM-CSF: 2 ng/mL
HSA: 5 mg/mL
pH: 7.2 – 7.4
Osmolality: 257-273 mOsm/kg
26 weeks shelf life / 7 day open bottle usage
Composition
Recombinant human GM-CSF
Sodium hyaluronate
HSA
Physiological salts
Energy Substrates
EDTA
Gentamicin sulfate
Phenol red
Essential amino acids
Non-essential amino acids
Antioxidants
Product codes
| Product Name | Product Code | Volume | Description |
|---|---|---|---|
| SAGE 1-StepTM GM-CSF | 77010003A | 3 mL | With HSA and phenol red |
Get In Touch With Us
We’d love to hear from you. How can we help?
Brochures, Catalogs & Flyers
Instructions for Use
Instructions for Use
GM-CSF: 2 ng/mL
HSA: 5 mg/mL
pH: 7.2 – 7.4
Osmolality: 257-273 mOsm/Kg
26 weeks shelf life / 7 day open bottle usage
Recombinant human GM-CSF
Sodium hyaluronate
HSA
Physiological salts
Energy Substrates
EDTA
Gentamicin sulfate
Phenol red
Essential amino acids
Non-essential amino acids
Antioxidants
- Hardy, K. & Spanos, S. (2002) Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol 172: 221-236
- Mor, G., Aldo, P. & Alvero, A. (2017). The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 17, 469–482 https://doi.org/10.1038/nri.2017.64
- Robertson, S.A. (2018) GM-CSF in IVF Embryo Culture. ART Scientific Edition 4: 1-4.
- Tevkin, S., Lokshin, V., Shishimorova, M. et al. (2014) The frequency of clinical pregnancy and implantation rate after cultivation of embryos in a medium with granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with preceding failed attempts of ART. Gynecol Endocrinol 30(Suppl 1): 9-12.
- Okabe-Kinoshita, M., Kobayashi, T., Shioya, M. et al. (2022) Granulocyte-macrophage colony- stimulating factor-containing medium treatment after thawing improves blastocyst-transfer outcomes in the frozen-thawed blastocyst-transfer cycle. J Assist Reprod Genet 39: 1373- 1381.
- Ziebe, S., Loft, A., Povlsen, B.B. et al. (2013) A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro fertilization. Fertil Steril 99: 1600-1609.
Procedures
Want unlimited First line support?
All our USA and Europe Customers get free unlimited first line support with a service contract.
Support & Compliance
Our global team is committed to providing the highest standards of service and support.

Batch Certificates
Use this tool to enter your batch number and download the corresponding certificate of analysis.

Service
We offer a range of contract options to suit your needs: preventative maintenance and service, reliable access to spare parts, product training, and online handling of service requests.
Get In Touch With Us
We’d love to hear from you. How can we help?