DuoRipe™
Cervical Ripening Balloon
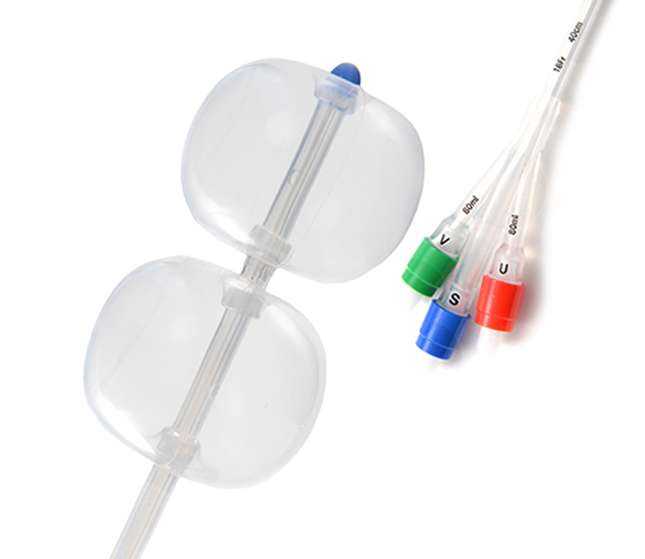
Designed to be Simple and Safe*
Intended to dilate the cervix and may improve labor outcomes.1-4
Healthcare providers can feel confident the device will facilitate induction in their patients.¹
Improved safety demonstrated by a lower likelihood of causing uterine hyperstimulation.²*

Has shown significantly reduced rates of fetal acidemia.⁵†

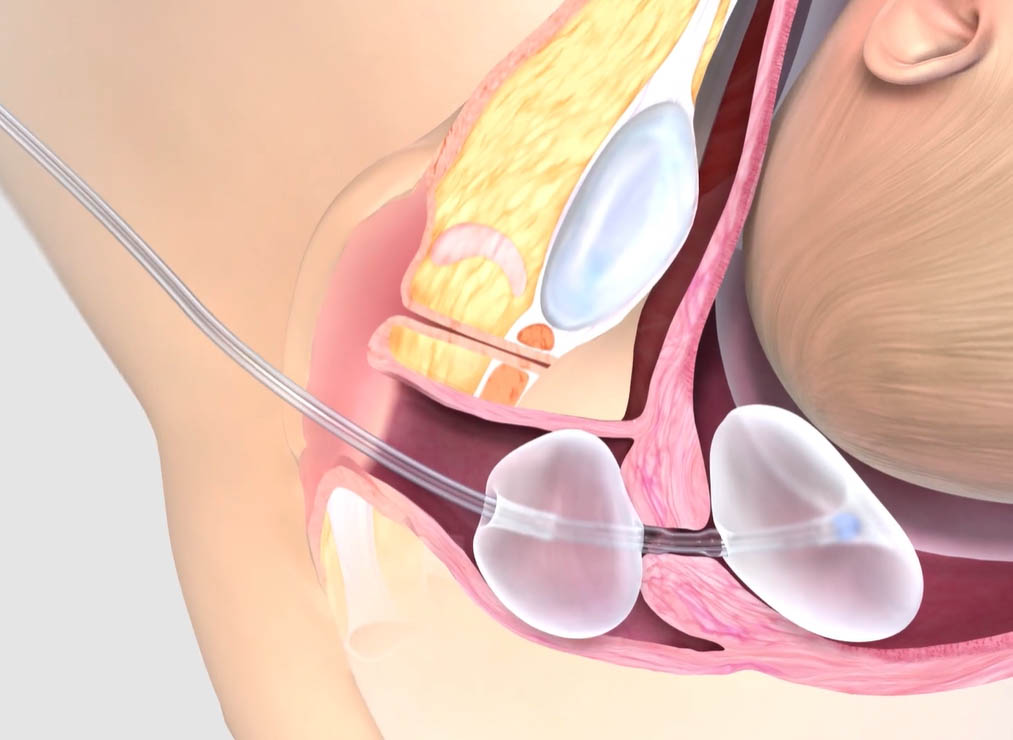
Constant pressure on both the internal and external os facilitates the process of gradual dilation.¹
Non-pharmacological1
Stylet for improved insertion1
Double-balloon advantage1
No traction required1
Cervical Ripening Balloon Key Features.
Product Codes
| Product Code | Reference Part Number | Description | Fr | Length | Balloon Volume |
|---|---|---|---|---|---|
| G48149 | J-CRB-184000 | Cervical Ripening Balloon | 18 | 40cm | 80 mL per balloon |
| G19891 | J-CRBS-184000 | Cervical Ripening Balloon with Stylet | 18 | 40cm | 80 mL per balloon |
Brochures, Catalogs and Flyers
Footnotes:
* (Compared to PGE2) prospective controlled trial, non-randomized. n=473.
† (Compared to PGE2) matched retrospective cohort study. n=854.
References:
- Cook Cervical Ripening Balloon with Stylet Instructions for Use. Bloomington, IN: Cook Medical; 2021.
- Wang L, Wang G, Cao W, et al. Comparison of the Cook vaginal cervical ripening balloon with prostaglandin E2 insert for induction of labor in late pregnancy. Arch Gynecol Obstet. 2020;302(3):579-584.
- Solt I, Frank Wolf M, Ben-Haroush S, et al. Foley catheter versus cervical double balloon for labor induction: a prospective randomized study. J Matern Fetal Neonatal Med. 2021;34(7):1034-1041.
- Hoppe KK, Schiff MA, Peterson SE, et al. 30 mL single- versus 80 mL double-balloon catheter for pre-induction cervical ripening: a randomized controlled trial. J Matern Fetal Neonatal Med. 2016;29(12):1919–1925.
- Brown J, Beckman M. Induction of labour using balloon catheter and prostaglandin gel. Aust N Z J Obstet Gynaecol. 2017;57(1):68-73.
- R&D Report Non-Clinical Bench Testing Study Report: Inflation, Dimensional, and Volume Recovery Testing of the Cook Inc. Cervical Ripening Balloon 2209043-04-R 2022.
IMPORTANT SAFETY INFORMATION
The Cervical Ripening Balloon with Stylet is a single use device, indicated for mechanical dilation of the cervical canal prior to labor induction at term when the cervix is unfavorable for induction.
CONTRAINDICATIONS: Patients planning to undergo exogenous prostaglandin administration; placenta previa, vasa previa or placenta percreta; transverse fetal orientation; prolapsed umbilical cord; prior hysterotomy, myomectomy or any other full-thickness uterine incision; pelvic structural abnormality; genital herpes infection; invasive cervical cancer; abnormal fetal heart rate; breech; maternal heart disease; multiple gestational pregnancy; polyhydramnios; presenting part above pelvic inlet; severe maternal hypertension; any contraindication to labor induction; ruptured membranes.
WARNINGS: Concomitant use of the Cervical Ripening Balloon with exogenous prostaglandins may increase the risk of adverse events associated with prostaglandin administration. The stylet should only be used to traverse the tip of the catheter through the cervix and be removed as soon as the uterine balloon is above the level of the internal os prior to full insertion of catheter. Aggressive insertion may result in injury to baby. Should not be left indwelling for longer than 12 hours. Always inflate with sterile saline; do not overinflate the balloon or use air, carbon dioxide, or any other gas. If spontaneous rupture of membranes occurs with the device in place, it is recommended both balloons be deflated and device to be removed due to risk of device becoming entangled in the umbilical cord which would necessitate an emergent cesarean delivery.
Consult the IFU prior to use of the Cervical Ripening Balloon, for detailed instructions and potential risks. https://coopersurgical.com/crbs-ifu




