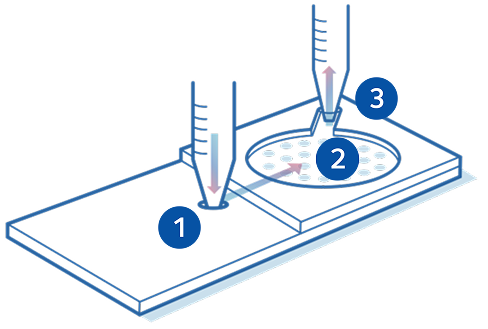
ZyMōt™ Multi Sperm
Separation Device

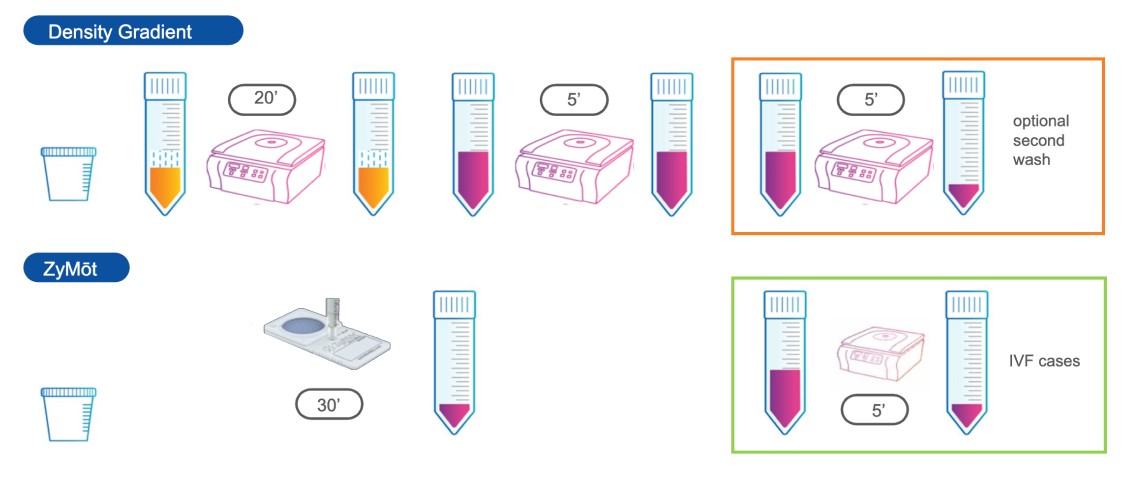
Sperm preparation made easy
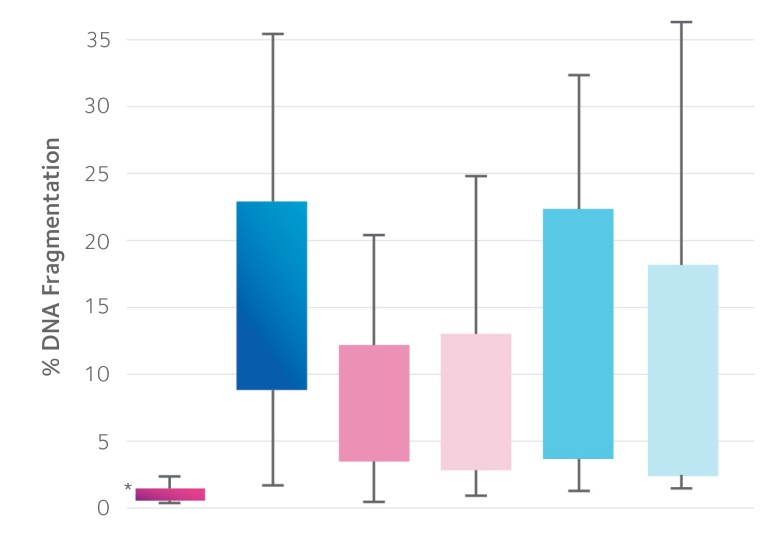
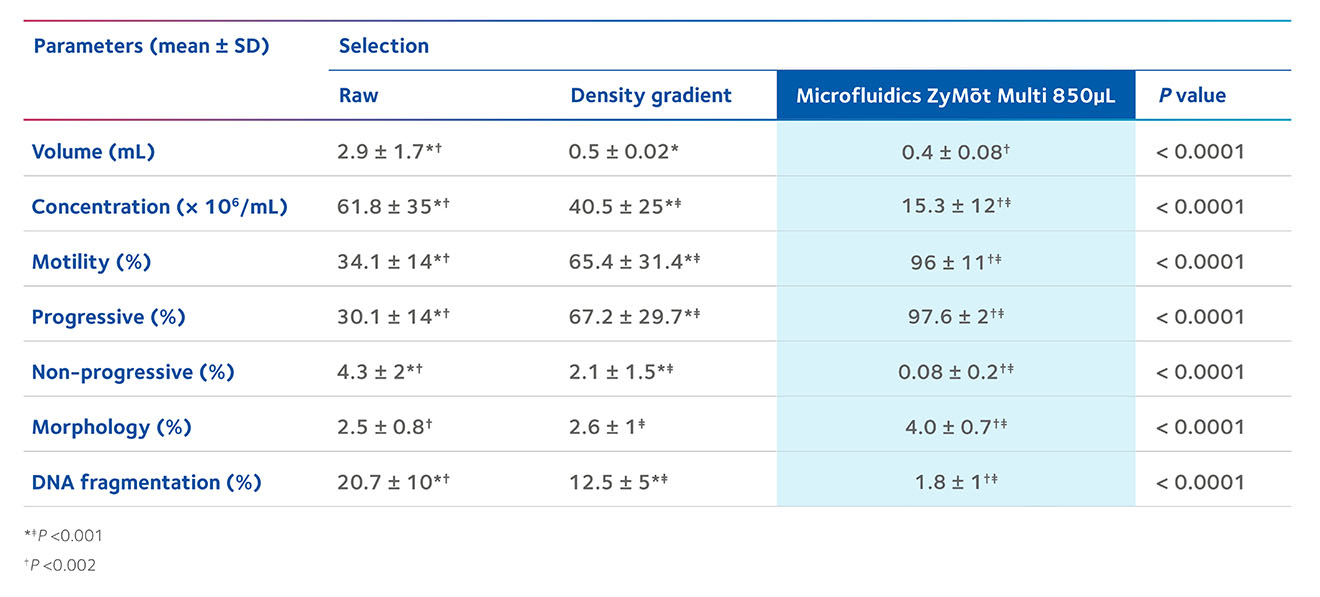
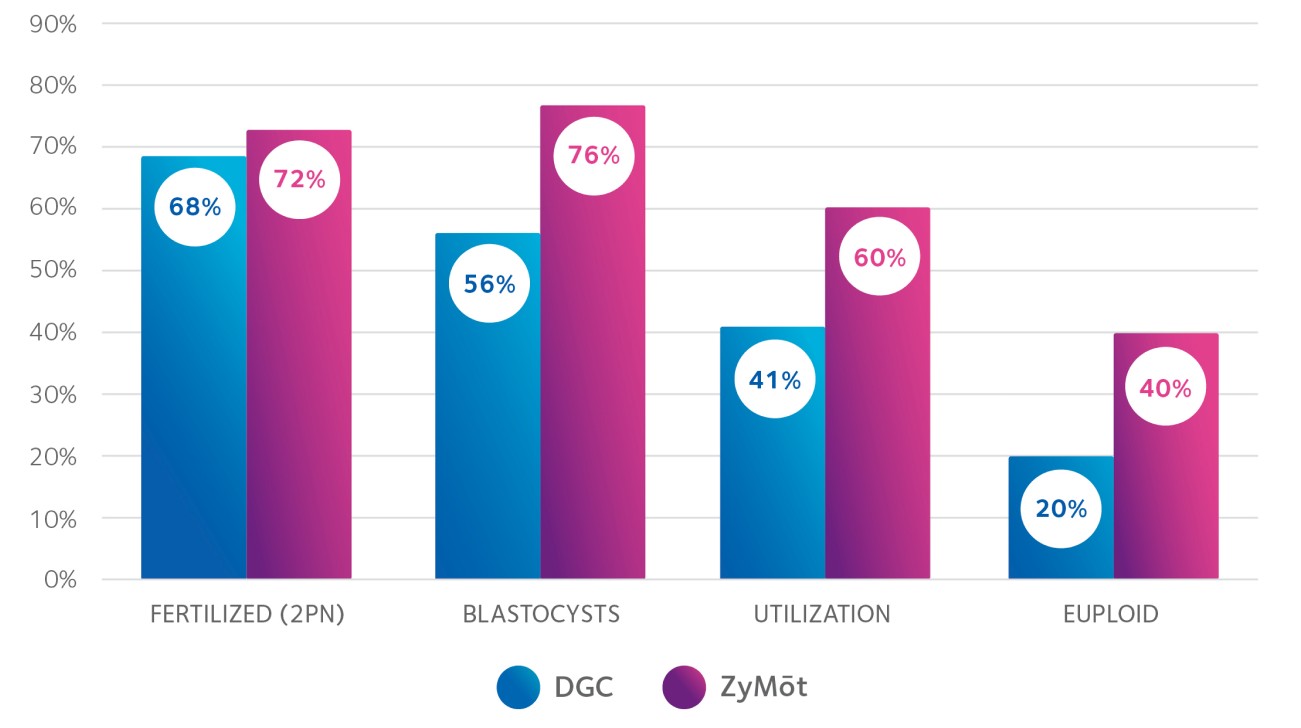
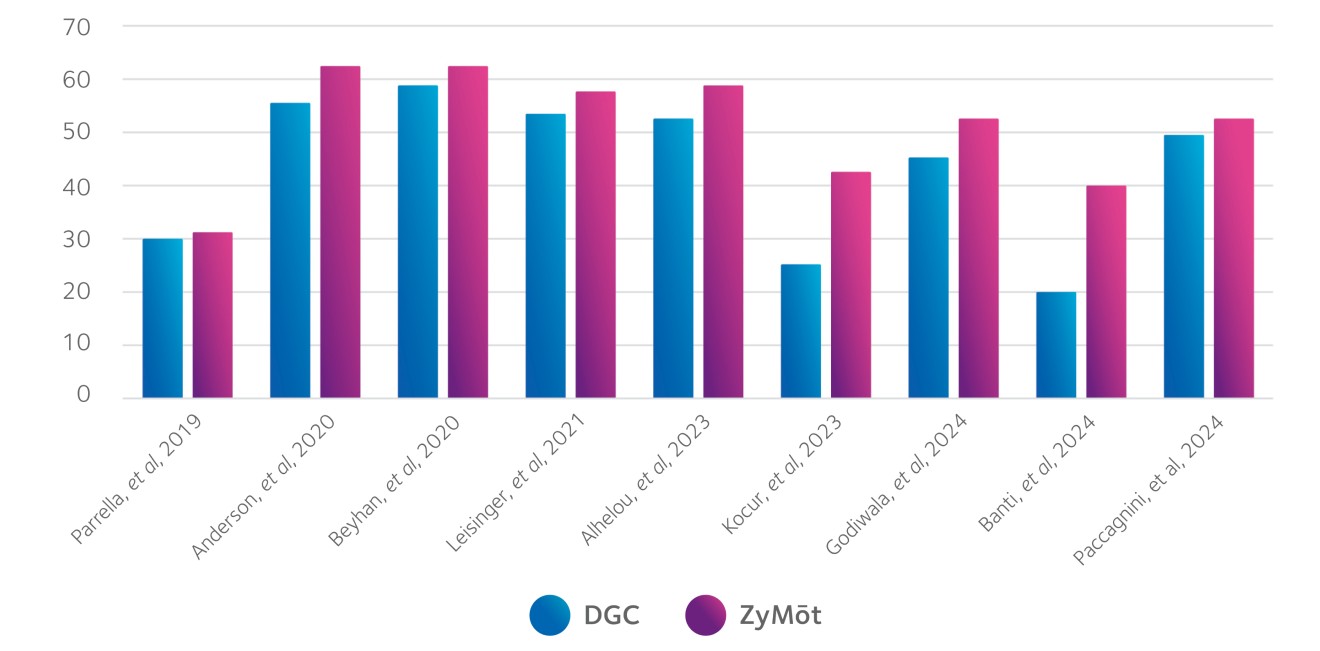
The ZyMōt™ Multi Sperm Separation Device is an innovative and simpler way to isolate high-quality, motile sperm for use in ART procedures, separating sperm with low DNA fragmentation1-4 – helping to increase the chance of a successful fertility treatment cycle.4–7
Product characteristics
Indications for use8
The ZyMōt™ Multi Sperm Separation Device is intended for preparing motile sperm from semen for use in the treatment of infertile couples by intracytoplasmic sperm injection (ICSI), in vitro fertilization (IVF), and intrauterine insemination (IUI) procedures.
Description8
• Manufactured in two processing volumes: 850μL and 3mL
• The primary difference between the devices is the processing volume
• Single-use only
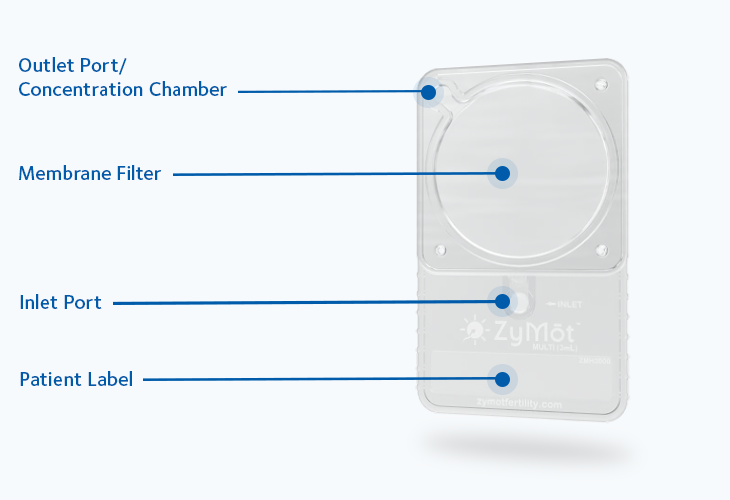
Mode of action
Sperm separation using ZyMōt™ allows for high-quality, motile sperm with low DNA fragmentation to pass through the microporous membrane, ready for collection.4,8