| Symbol | Standard Reference | Symbol Title | Explanatory Text |
|---|---|---|---|
 | ISO 15223-1 Reference no.5.1.6 | Catalogue Number | Indicates the manufacturer’s catalogue number so that the medical device can be identified. |
 | ISO 15223-1 Reference no.5.1.5 | Batch Code | Indicates the manufacturer’s batch code so that the batch or lot can be identified. |
 | ISO 15223-1 Reference no.5.1.7 | Serial number | Indicates the manufacturer’s serial number so that a specific medical device can be identified |
 | ISO 15223- 1 Reference no.5.1.11 | Country of Manufacture | To identify the country of manufacture of products. Country of manufacture (“CC” shall be replaced by either the two letter or three letter country code) |
 | ISO 15223-1 Reference no.5.1.3 | Date of manufacture | Indicates the date when the medical device was manufactured. |
 | ISO 15223-1 Reference no.5.1.4 | Use-by date | Indicates the date after which the medical device is not to be used. |
 | ISO 15223- 1 Reference no.5.7.7 | Medical device | Indicates the item is a medical device. |
 | ISO 15223-1 Reference no.5.1.2 | Authorized representative in the European Community/European Union | Indicates the authorized representative in the European Community / European Union. |
 | ISO 15223-1 Reference no.5.2.3 | Sterilized using ethylene oxide | Indicates a medical device that has been sterilized using ethylene oxide. |
 | ISO 15223-1 Reference no.5.2.4 | Sterilized using irradiation | Indicates a medical device that has been sterilized using irradiation. |
 | ISO 15223-1 Reference no.5.2.1 | Sterile | Indicates a medical device that has been subjected to a sterilization process. |
 | ISO15223-1 Reference no.5.2.2 | Sterilized using aseptic processing techniques | Indicates a medical device that has been manufactured using accepted aseptic techniques. |
 | ISO 15223-1 Reference no.5.2.5 | Sterilized using steam or dry heat | To indicate that the device is provided sterile and has been sterilized using steam or dry heat. |
 | ISO 15223-1 Reference no.5.2.9 | Sterile fluid path | Indicates the presence of a sterile fluid path within the medical device in cases when other parts of the medical device, including the exterior, might not be supplied sterile. |
 | ISO 15223-1 Reference no.5.2.10 | Sterilized using vaporized hydrogen peroxide | Indicates a medical device that has been sterilized using vaporized hydrogen peroxide. |
 | ISO 15223-1 Reference no.5.2.6 | Do not resterilize | Indicates a medical device that is not to be resterilized. |
 | ISO 15223-1 Reference no.5.2.7 | Non-sterile | Indicates a medical device that has not been subjected to a sterilization process. |
 | ISO 15223-1 Reference no.5.4.2 | Do not re-use | Indicates a medical device that is intended for one single use only. |
 | ISO 15223-1 Reference no.5.2.8 | Do not use if package is damaged and consult instructions for use | Indicates a medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions for use for additional information. |
 | ISO 15223-1 Reference no.5.4.4 | Caution | Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator awareness or operator action in order to avoid undesirable consequences. |
 | ISO 15223-1 Reference no.5.4.3 | Consult instructions for use | Indicates the need for the user to consult the instructions for use. |
 | ISO 15223-1 Reference no.5.1.1 | Manufacturer | Indicates the medical device manufacturer. |
 | ISO 15223-1 Reference no.5.1.8 | Importer | Indicates the entity importing the medical device into the locale. |
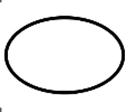 | ISO 15223-1 Reference no.5.2.11 | Single sterile barrier system | Indicates a single sterile barrier system. |
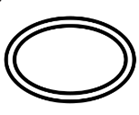 | ISO 15223-1 Reference no.5.2.12 | Double sterile barrier system | Indicates two sterile barrier systems. |
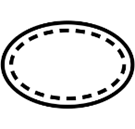 | ISO 15223-1 Reference no.5.2.13 | Single sterile barrier system with protective packaging inside | Indicates a single sterile barrier system with protective packaging inside. |
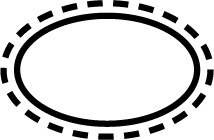 | ISO 15223-1 Reference no.5.2.14 | Single sterile barrier system with protective packaging outside | Indicates a single sterile barrier system with protective packaging outside. |
 | ISO 15223-1 Reference no.5.1.10 | Model number | To identify the model number or type number of a product. |
| ISO 15223-1 Reference no.5.3.1 | Fragile, handle with care | Indicates a medical device that can be broken or damaged if not handled carefully. | |
 | ISO 15223-1 Reference no.5.3.2 | Keep away from sunlight | Indicates a medical device that needs protection from light sources. |
 | ISO 15223-1 Reference no.5.3.4 | Keep dry | Indicates a medical device that needs protection from moisture. |
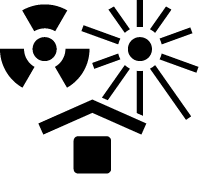 | ISO 15223-1 Reference no.5.3.3 | Protect from heat and radioactive sources | Indicates a medical device that needs protection from heat and radioactive sources. |
 | ISO 15223-1 Reference no.5.3.5 | Lower limit of temperature | Indicates the lower limit of temperature to which the medical device can be safely exposed. |
 | ISO 15223-1 Reference no.5.3.6 | Upper limit of temperature | Indicates the upper limit of temperature to which the medical device can be safely exposed. |
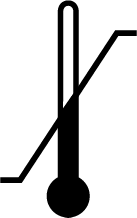 | ISO 15223-1 Reference no.5.3.7 | Temperature limit | Indicates the temperatures to which the medical device can be safely exposed. |
 | ISO 15223-1 Reference no.5.3.8 | Humidity limitation | Indicates the range of humidity to which the medical device can be safely exposed. |
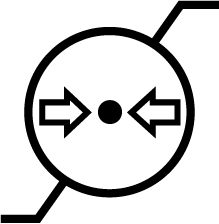 | ISO 15223-1 Reference no.5.3.9 | Atmospheric pressure limitation | To indicate the acceptable upper and lower limits of atmospheric pressure for transport and storage. |
 | ISO 15223-1 Reference no.5.5.1 | In vitro diagnostic medical device | Indicates a medical device that is intended to be used as an in vitro diagnostic medical device. |
 | ISO 15223-1 Reference no.5.4.6 | Contains human blood or plasma derivatives | Indicates a medical device that contains or incorporates human blood products or plasma derivatives. |
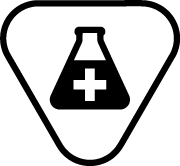 | ISO 15223-1 Reference no.5.4.7 | Contains a medicinal substance | Indicates a medical device that contains or incorporates a medicinal substance. |
 | ISO 15223-1 Reference no 5.4.8 | Contains biological material of animal origin | Indicates a medical device that contains biological tissue, cells, or their derivatives, of animal origin. |
| ISO 15223-1 Reference no.5.5.6 | For IVD performance evaluation only | Indicates an IVD device that is intended to be used only for evaluating its performance characteristics before it is placed on the market for medical diagnostic use. | |
 | ISO 15223-1 Annex B.2 Reference no.5.4.5 | Not made with natural rubber latex | Indicates that natural rubber latex was not used in the manufacturing of the product, its container, or its packaging. |
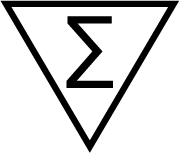 | ISO 15223-1 Reference no.5.5.5 | Contains sufficient for <n> tests | Indicates the total number of IVD tests that can be performed with the IVD medical device. |
 | ISO 15223-1 Reference no.5.6.1 | Sampling site | Indicates a medical device or blood processing application that includes a system dedicated to the collection of samples of a given substance stored in the medical device or blood container. |
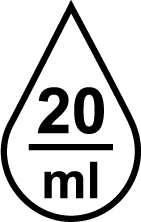 | ISO 15223-1 Reference no.5.6.4 | Drops per millilitre | Indicates the number of drops per millilitre. |
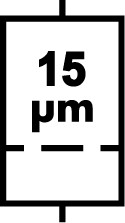 | ISO 15223-1 Reference no.5.6.5 | Liquid filter with pore size | Indicates an infusion or transfusion system of the medical device that contains a filter of a particular nominal pore size. |
 | ISO 15223-1 Reference no.5.4.10 | Contains hazardous substances | Indicates a medical device that contains substances that can be carcinogenic, mutagenic, reprotoxic (CMR), or substances with endocrine disrupting properties. |
 | ISO 15223-1 Reference no.5.4.5 | Contains or presence of natural rubber latex | Indicates the presence of dry natural rubber or natural rubber latex as a material of construction within the medical device or the packaging of a medical device. |
 | ISO 15223-1 Reference no.5.6.3 | Non-pyrogenic | Indicates a medical device that is non-pyrogenic. |
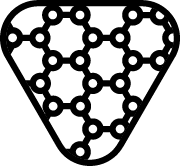 | ISO 15223-1 Reference no.5.4.11 | Contains nano materials | Indicates a medical device that contains nano materials. |
 | ISO 15223-1 Reference no.5.4.1 | Biological risks | Indicates that there are potential biological risks associated with the medical device. |
| ISO 15223-1 Reference no.5.5.2 | Control | Indicates a control material that is intended to verify the performance of another medical device. | |
| ISO 15223-1 Reference no.5.5.3. | Negative control | Indicates a control material that is intended to verify the results in the expected negative range | |
| ISO 15223-1 Reference no.5.5.4 | Positive control | Indicates a control material that is intended to verify the results in the expected positive range. | |
 | ISO 15223-1 Reference no.5.6.6 | One-way valve | Indicates a medical device with a valve that allows flow in only one direction. |
 | ISO 15223-1 Reference no.5.7.1 | Patient number | Indicates a unique number associated with an individual patient. |
 | ISO 15223-1 Reference no.5.7.2 | Patient name | Indicates the name of the patient. |
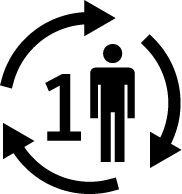 | ISO 15223-1 Reference no.5.4.12 | Single patient-multiple use | Indicates a medical device that may be used multiple times (multiple procedures) on a single patient. |
 | ISO 15223-1 Reference no.5.7.3 | Patient identification | Indicates the identification data of the patient. |
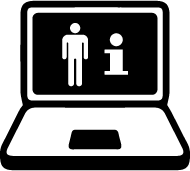 | ISO 15223-1 Reference no.5.7.4 | Patient information website | Indicates a website where a patient may obtain additional information on the medical product. |
 | ISO 15223-1 Reference no.5.7.5 | Health care centre or doctor | Indicates the address of the health care centre or doctor where medical information about the patient may be found. |
 | ISO 15223-1 Reference no.5.7.6 | Date | To identify the date that information was entered, or a medical procedure took place. |
 | ISO 15223-1 Reference no.5.7.8 | Translation | To identify that the original medical device information has undergone a translation which supplements or replaces the original information. |
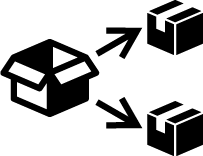 | ISO 15223-1 Reference no.5.7.9 | Repackaging | To identify that a modification to the original medical device packaging configuration has occurred. |
 | ISO 15223-1 Reference no.5.1.9 | Distributor | Indicates the entity distributing the medical device into the locale. |
 | ISO 15223-1 Reference no.5.7.10 | Unique device identifier | Indicates a carrier that contains unique device identifier information. |
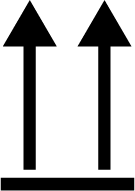 | ISO 7000 Reference no.0623 | This way up | To indicate correct upright position of the transport package. |
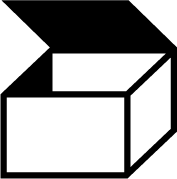 | ISO 7000 Reference no.2794 | Packaging unit | To indicate the number of pieces in the package. |
 | ISO 7000 Reference no.2723 | Non-pyrogenic, fluid path | On medical devices: to indicate that the fluid path is non-pyrogenic. |
 | BS EN 15986:2001 Reference no. Clause 4.2 | Contains or presence of phthalate | Medical device is derived from or manufactured from products containing phthalate. |
 | IEC 60417 Reference no.5840 | Type B applied part | To identify a type B applied part complying with, IEC 60601-1. |
 | IEC 60417 Reference no.5333 | Type BF applied part | To identify a type BF applied part complying with IEC 60601-1. |
 | IEC 60417 Reference no.5335 | Type CF applied part | To identify a type CF applied part complying with IEC 60601-1. |
 | IEC 60417 Reference no.5841 | Defibrillation-proof type B applied part | To identify a defibrillation-proof type B applied part complying with IEC 60601-1. |
 | IEC 60417 Reference no.5334 | Defibrillation-proof type BF applied part | To identify a defibrillation-proof type BF applied part complying with IEC 60601-1 |
 | IEC 60417 Reference no.5336 | Defibrillation-proof type CF applied part | To identify a defibrillation-proof Type CF applied part complying with IEC 60601-1. |
 | ISO 7010 Reference no.W012 | Warning; Electricity | To warn of electricity. |
| IEC 60417 Reference no.5036 | Dangerous voltage | To indicate hazards arising from dangerous voltages. | |
 | Reference no. ISO 7010_WOO1 | General warning sign | To signify a general warning. |
| IEC 60417 Reference no.5032 | Alternating current | To indicate on the rating plate that the equipment is suitable for alternating current only; to identify relevant terminals. | |
 | IEC 60417 Reference no.5031 | Direct Current | To indicate on the rating plate that the equipment is suitable for direct current only; to identify relevant terminals. |
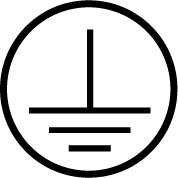 | IEC 60417 Reference no.5019 | Protective earth; protective ground | To identify any terminal which is intended for connection to an external conductor for protection against electric shock in case of a fault, or the terminal of a protective earth (ground) electrode. |
 | IEC 60417 Reference no.5017 | Earth; ground | To identify an earth (ground) terminal in cases where neither the symbol 5018 nor 5019 is explicitly required. |
 | IEC 60417 Reference no.5021 | Equipotentiality | To identify the terminals which, when connected together, bring the various parts of an equipment or of a system to the same potential, not necessarily being the earth (ground) potential, e.g. for local bonding. |
 | IEC 60417 Reference no.5172 | CLASS II equipment | To identify equipment meeting the safety requirements specified for Class II equipment according to IEC 61140. |
 | IEC-60417 Reference no.5134 | Electrostatic sensitive devices | To indicate packages containing electrostatic sensitive devices, or to identify a device or a connector that has not been tested for immunity to electrostatic discharge. |
 | IEC 60417 Reference no.5140 | Non-ionizing electromagnetic radiation | To indicate generally elevated, potentially hazardous, levels of non-ionizing radiation, or to indicate equipment or systems e.g. in the medical electrical area that include RF transmitters of that intentionally apply RF electromagnetic energy for diagnosis or treatment. |
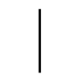 | IEC 60417 Reference no.5007 | “ON” (power) | To indicate connection to the mains, at least for mains switches or their positions, and all those cases where safety is involved. |
 | IEC 60417 Reference no.5008 | “OFF” (power) | To indicate disconnection from the mains, at least for mains switches or their positions, and all those cases where safety is involved. |
 | IEC 60417 Reference no.5264 | “ON” for a part of equipment | To indicate the “ON” condition for a part of equipment, if the symbol 5007 cannot be used, for example, to identify the “ON” position of a switch. |
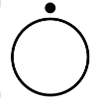 | IEC 60417 Reference no.5265 | “OFF” for a part of equipment | To indicate the “OFF” condition for a part of equipment, if the symbols 5008 cannot be used, for example, to identify the “OFF” position of a switch. |
 | IEC 60417 Reference no.5266 | Stand-by or preparatory state for a part of equipment | To indicate the stand-by or preparatory state for a part of equipment, if the symbol 5009 cannot be used, for example, to identify the “STAND-BY” position of a switch. |
 | IEC 60417 Reference no.5005 | Plus; positive polarity | To identify the positive terminal(s) of equipment which is used with, or generates direct current. |
 | IEC 60417 Reference no.5006 | Minus; negative polarity | To identify the negative terminal(s) of equipment which is used with, or generates direct current. |
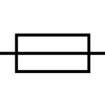 | IEC 60417-1 Reference no.5016 | Fuse | To identify fuse boxes or their location. |
 | ASTM F2503 Reference no. Table 2; 7.4.6.1. Fig.6,7 | MR Conditional | An item with demonstrated safety in the MR environment within defined conditions. |
 | ASTM F2503 Reference no. Table 2, Symbol 7.3.3. 7.4.9.1; Fig. 9 | MR Unsafe | 3.1.14: An item which poses unacceptable risks to the patient, medical staff or other persons within the MR environment. |
 | IEC 60601-1, Reference no. ISO 7010-M002 | Refer to instruction manual/ booklet | To signify that the instruction manual/booklet must be read. |
 | N/A | Directs health care practitioner to peel open a package. | N/A |
| IEC 60417 Reference no. ISO 7000 3650 | Universal Serial Bus (USB), port/plug | To identify a port or plug as meeting the generic requirements of the Universal Serial Bus (USB). To indicate that the device is plugged into a USB port or is compatible with a USB port | |
 | ISO 7000 Reference no.3079 | Open Here | To identify the location where the package can be opened and indicate the method of opening it. |
 | IEC 60417 Reference no. ISO 7000 1135 | General symbol for recovery recyclable | To indicate that the marked item or its material is part of a recovery or recycling process. |
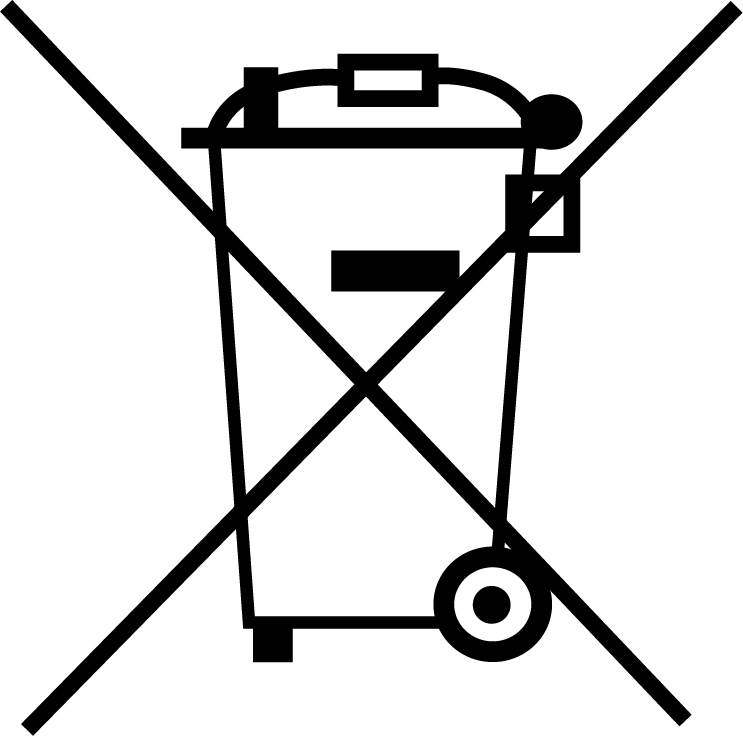 | EN 50419 EU Directive 2012/19/EU | WEEE (Waste electrical and Electronic Equipment) 2005 | Indicates separate collection for waste of electrical and electronic equipment manufactured prior to 13 August 2005. |
 | EN 50419 EU Directive 2012/19/EU | WEEE (Waste electrical and Electronic Equipment) | Indicates separate collection for waste of electrical and electronic equipment. |
 | N/A | UkrSEPRO conformity mark | N/A |
 | N/A | Canadian and US Certification mark | N/A |
 | 21 CFR Part 15 | Federal Communications Commission | Meets FCC requirements per 21 CFR Part 15. |
| 21 CFR 801.109 | Prescription devices | Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare practitioner. | |
| N/A | Product conforms to the Medical Device Directive 93/42/EEC | Signifies European technical conformity. | |
 | N/A | Use within “X” days of opening | N/A |
 | US: 29 CFR 1910:1200 (HCS) EU: Regulation 1272/2008/EC GHS 07 | Health hazards | Indicates a potential for health risk to the user of the medical device. |
REV. 01 – 01/2025
