PGT-A

Unique innovation to improve the odds of pregnancy and IVF success
The only test using artificial intelligence (AI) based on clinical outcome data, eliminating subjectivity, and improving accuracy.
Using two independent analyses (CNV & SNP) to check for abnormalities, providing greater confidence in robust and accurate results.
Availability
This product is available for sale in selected countries around the world.

>13% increase in ongoing pregnancy and live birth rates vs standard PGT-A¹

Emerging Evidence
Patients’ chances of having a baby improved with the PGTaiSM 2.0 Platform! A single-center study shows the innovative PGTai 2.0 platform helped patients fulfill their ultimate dream of having a baby by increasing ongoing pregnancy and live birth rates.

Introducing PGT-A with PTA
We are implementing Primary Template-directed Amplification (PTA), a novel approach to DNA amplification for embryo biopsy samples.
This innovation represents the first major advancement to DNA amplification since 2009 and marks a milestone in improving the quality of DNA amplification in embryo samples.
This change is being implemented because CooperSurgical Genomics, hopes to provide the most advanced technology available to you and your patients which means from time-to-time upgrades to our technology require that we permanently discontinue outdated processes.
If you have any queries or would like any further information please click here to contact your Territory Account Manager or Clinical Support Specialist.
There are no required changes to your clinical processes.
No, there is no added cost to PGT-A testing.
No, at this time we do not anticipate any changes to our key performance metrics.
No, there will not be any changes to the report format or delivery.
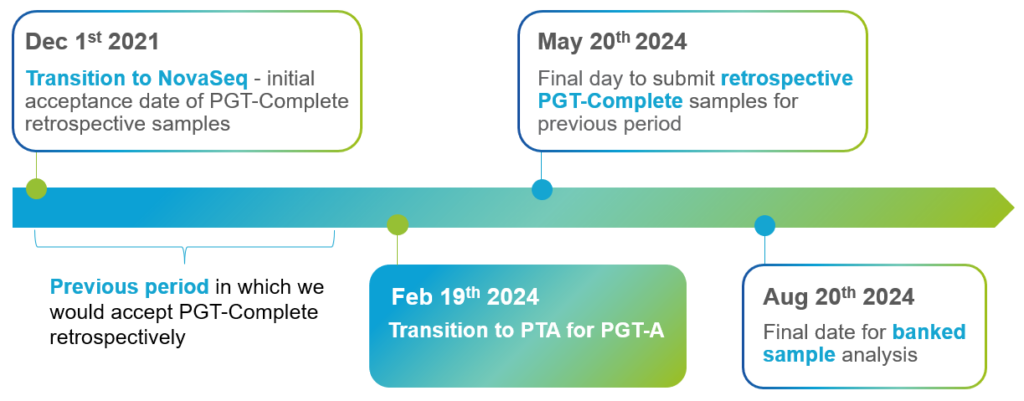
No, only samples received after February 19th 2024, will be processed by PTA
There will be a grace period of 90 days (up to May 20th 2024) to submit buccal swabs and documentation related to any PGT-A cases that were analyzed between the dates of Dec 1st 2021 and Feb 19th 2024. During this period our laboratory staff will be happy to conduct retrospective PGT-Complete.
YES! Once we have transitioned to the new processes any cases received after Feb 19th 2024 will have the option of conducting PGT-Complete retrospectively.
Remember, PGT-A cases submitted between Dec 1st 2021 and Feb 19th 2024 will NO longer be eligible for retrospective PGT-Complete after May 20th 2024.
We transitioned amplification for PGT-M cases to PTA starting in Feb of 2023. Only older cases, or those otherwise amplified by a different technology are impacted.
We are providing a 6-month period (until Aug 20th 2024) for our partners to finalize analysis of any embryos held in storage at our CooperGenomics® locations.
Laboratory Process Improvement Timeline
Don’t forget to mark your calendar with these important dates
Excluding New York State samples and samples sent to our UK-based laboratory
NIPGT-A Research Update
Our dedication to investigation and innovation expands to non-invasive methodologies
Our Overview of the paper:
1000 Mosaic Embryo Transfers Viotti et al, 2021
Clinic Testimonials
At CARE Fertility, we have been impressed with the transition from standard to PGTai; the staff education and clarity of reporting. In addition, we are reassured by the increased precision of this test and believe it brings benefits for our patients.
Alison Campbell
Director of Embryology, CARE Fertility Group, London, UK
The switch to PGTai has more than halved the turnaround time on our results, with the lab now averaging 3 days from sample receipt to providing us with the final report. That’s been an enormous benefit, those extra few days waiting for results make a huge difference to our patients! We’ve seen a drop in the number of ‘no call’ results since the changeover to below 2%. Most importantly the level of service from the staff we deal with in the UK laboratory has been outstanding, always friendly, helpful and on top of anything we need.
Kelli Sorby
Scientific Director, Number1 Fertility, Melbourne, Australia
Get In Touch With Us
We’d love to hear from you. How can we help?
Brochures, Catalogs & Flyers
Reproductive Genetic Testing Brochure
Reproductive Genetic Testing Brochure
PGT-Complete Brochure
PGT-Complete Brochure
PGT-A Patient Brochure
PGT-A Patient Brochure
PGT-A Clinician Brochure
PGT-A Clinician Brochure
PGTai White Paper
PGTai White Paper
PGTai 2.0 Flyer
PGTai 2.0 Flyer
PGTai Flyer
PGTai Flyer
1. Buldo-Licciardi J, Large M, McCulloh D, McCaffrey C, Grifo J. Second generation artificial intelligence technology for preimplantation genetic testing (PRT) improves pregnancy outcomes in single thawed euploid embryo transfer cycles (STEET). Presented at American Society for Reproductive Medicine on October 19, 2020.
Available at: https://asrm.confex.com/asrm/2020/meetingapp.cgi/Paper/8645. Accessed November 2, 2021. 2. CooperGenomics, internal data on file.
Procedures
Want unlimited First line support?
All our USA and Europe Customers get free unlimited first line support with a service contract.
Support & Compliance
Our global team is committed to providing the highest standards of service and support.

Batch Certificates
Use this tool to enter your batch number and download the corresponding certificate of analysis.

Service
We offer a range of contract options to suit your needs: preventative maintenance and service, reliable access to spare parts, product training, and online handling of service requests.
Get In Touch With Us
We’d love to hear from you. How can we help?